Schaudinn’s Fixative is a mercuric chloride-based fluid that is used to preserve the integrity of sample specimens in preparation for analysis. Once specimen fixation occurs, a stain is usually applied to the samples to help in the identification of specific microscopic organisms.
While not a complete list, the fields of Parasitology, Cytology and Fecal Sampling commonly use Schaudinn’s Fixative on samples in preparation for microscopy.
Mercuric chloride and ethyl alcohol (ethanol) are usually the primary ingredients that make up Schaudinn’s Fixative. Varying mixtures of the fixative sometimes include acetic acid, glycerin, isopropanol, and methyl alcohol.
Exposure Limits
The following chart outlines the occupational exposure limits for the chemicals commonly found in Schaudinn’s Fixative.
| Chemical | OSHA PEL | NIOSH REL |
| Acetic Acid | 10 ppm TWA | 10 ppm TWA |
| Ethyl Alcohol | 1,000 ppm TWA | 1,000 ppm TWA |
| Glycerin (Mist) | 5 mg/m3 TWA (respiratory) | None Listed |
| Isopropanol | 400 ppm TWA | 400 ppm TWA |
| Mercuric Chloride | 0.1 mg/m3 Ceiling (listed under Mercury, aryl and inorganic compounds) | 0.05 mg/m3 TWA(listed under mercury compounds) |
| Methyl Alcohol | 200 ppm TWA | 200 ppm TWA |
MSDS Safety Statements1,2
We looked at the Material Safety Data Sheets for Schaudinn’s Fixative provided by two different brands. Upon inspection we found the following respiratory-related safety statements as well as possible health concerns from exposure.
- Toxic by inhalation, absorption or ingestion. Cannot be made nontoxic. Causes CNS depression, headache, intoxication, dilation of the pupils, convulsions nausea, and dizziness. Unconsciousness and death may result. Methanol intoxication may produce visual disturbances and blindness. Mercury salts are extremely toxic. Mercuric chloride is an experimental teratogen and mutagen. Signs of overexposure include increased salivation, foul breath, abdominal pain, bloody diarrhea and inflammation and/or ulceration of the mucous membranes.
- Causes respiratory tract irritation. May cause central nervous system effects including vertigo, anxiety, depression, muscle incoordination, and emotional instability. May cause gastrointestinal effects including gum and mouth inflammation, jaw necrosis, and loosening of the teeth. Acute exposure to high concentrations of mercury vapors may cause severe respiratory tract irritation.
- Do not breathe dust, mist, or vapor. Do not get in eyes, on skin, or on clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Do not ingest or inhale. Use only in a chemical fume hood.
- Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits.
Comments from a Chemist
 Sentry Air’s Mr. Luke Turner is a Ph.D. Chemist and technical sales associate. We often turn to Luke for his expertise and experience and asked that he weigh in on the staining and fixative process, along with likely exposure concerns:
Sentry Air’s Mr. Luke Turner is a Ph.D. Chemist and technical sales associate. We often turn to Luke for his expertise and experience and asked that he weigh in on the staining and fixative process, along with likely exposure concerns:
“In considering the application of mercuric chloride as a fixative, the saturated HgCl2 solution is susceptible to decomposition, particularly with reduction of the Hg2+ to elemental mercury (Hg0) in the presence of metals, biological functional groups and other substrates.3 Elemental mercury possesses a significant vapor pressure and should be captured to avoid user exposure and environmental accumulation. Sentry Air Systems mercury filter media provides effective capture of mercury vapor and is recommended for applications involving mercury compounds. The usage of mercuric chloride as a fixative is often accompanied by a subsequent treatment with Lugol’s reagent, a solution of iodine and potassium iodide.4 The Lugol’s reagent liberates mercuric chloride from tissue samples via conversion to mercuric iodide, which is readily solubilized in the aqueous medium. The Lugol’s reagent presents a user health concern due to the sublimation of iodine under ambient conditions. Sentry Air Systems provides specialty filter media for efficient capture of iodine vapor evolved during processes employing Lugol’s reagent. To eliminate any residual iodine following the Lugol’s reagent treatment, sodium thiosulfate is usually added for reduction to a stable iodide salt. Since the reaction is accompanied by formation of sulfur dioxide, filtration of this toxic gas is required to ensure a safe user breathing environment. Sentry Air Systems in-house testing has confirmed the suitability of acid gas filter media for the efficient capture of sulfur dioxide gas.5 Depending on the solution chemistry of the chosen Schaudinn’s fixative mixture, as well as the quenching reagents, a mixture of filter media may be used to facilitate effective filtration.”
It is clear that proper respiratory protection and ventilation measures be used while handling fixative and reagent solutions in order to avoid over exposure.
Containment Solutions
Sentry Air Systems has an extensive line of Laboratory and Chemical fume extraction systems. These systems are modular and can be customized to suit your particular application.
Our technical sales specialists take the time to fully understand your process and exposure concerns, so the appropriate filter media combination can be recommended.
As Mr. Turner said, our granule activated carbon media is especially unique in that we have the capability to blend specific amounts of impregnated carbon media to effectively capture certain chemicals and gases.
For example, a unique blend of our mercury, acid gas and iodine filter media may prove effective in the adsorption of harmful fixative and reagent vapor.
Sentry Air Systems also has the ability to customize the majority of our standard units. The images below depict actual custom units we created for customers.

Standard 40” W Exhaust Hood with custom microscope lens cutout on lid.
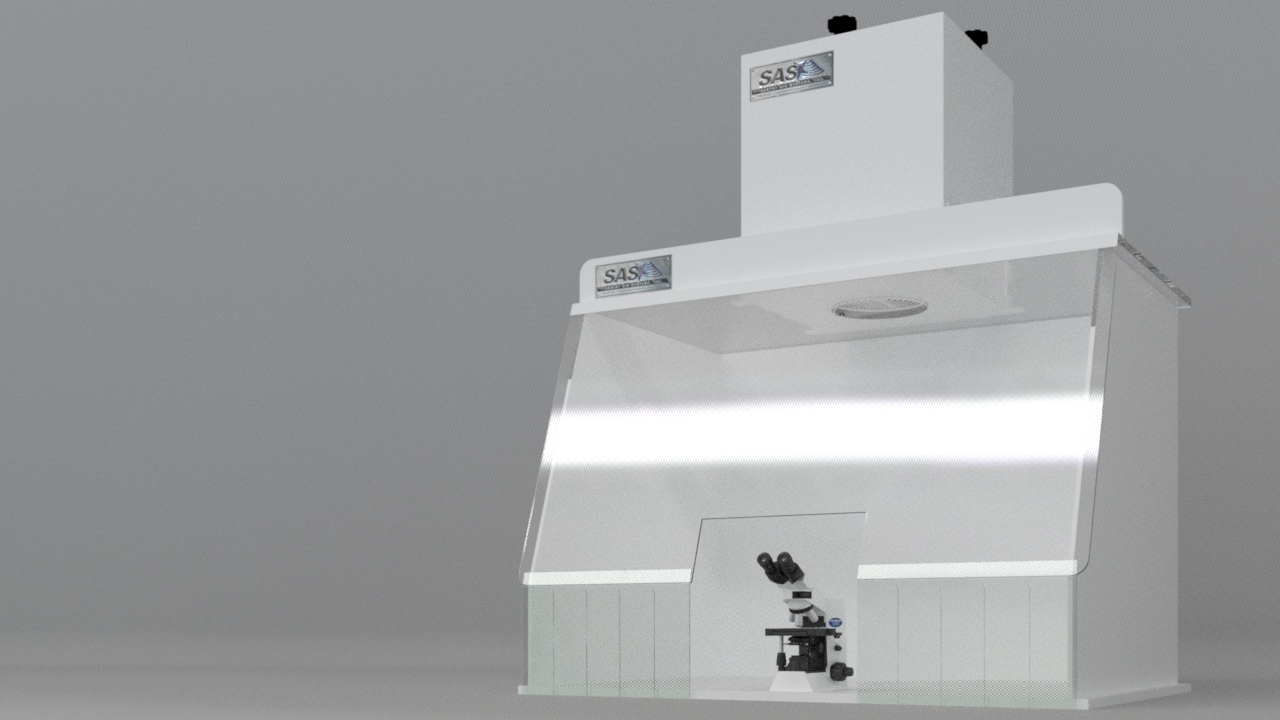
Standard 30” W Ductless Containment Hood with custom cutout for microscope placement.
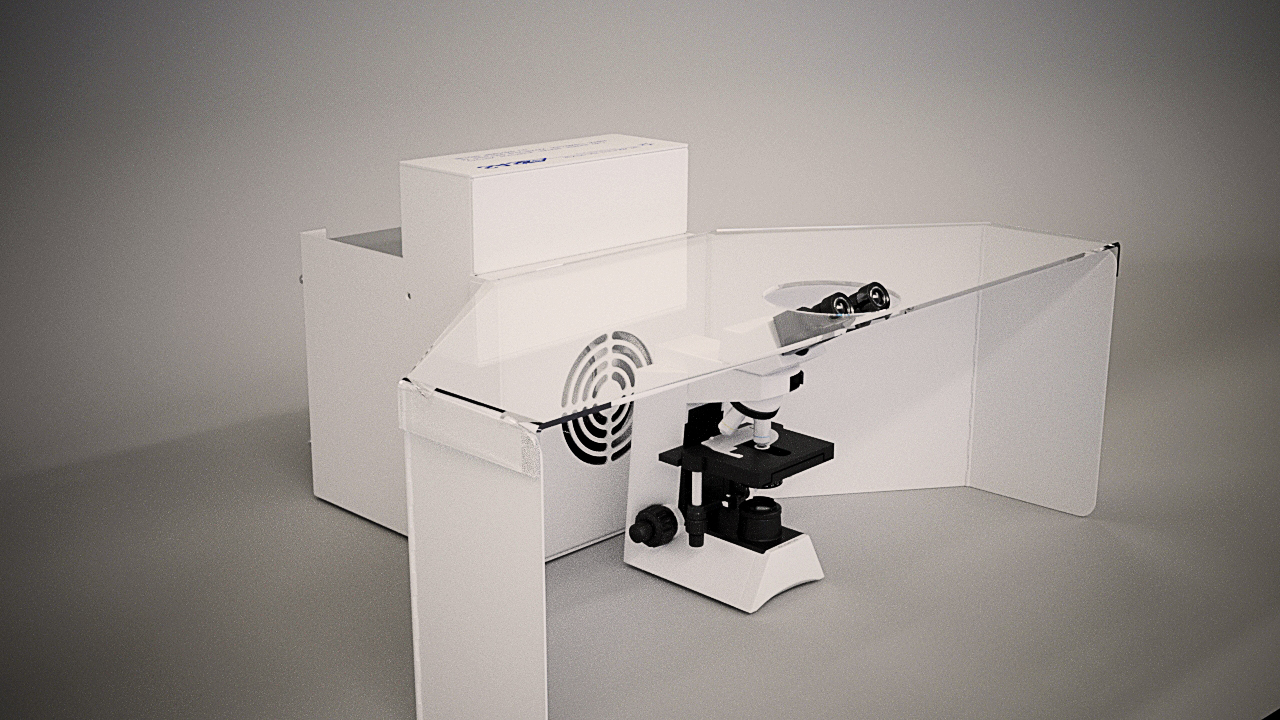
Standard Model 300 Winged Sentry with custom microscope lens cutout on lid.
Read our earlier blog post about fecal fume containment in a lab setting.
Contact Us
For more information or to receive a free quote, contact Sentry Air Systems at 800.799.4609, email sales@sentryair.com, visit our website or fill out the feedback form below.
Sources
1Medical Chem Corp. MSDS For Schaudinn’s Fixative
2Remel Material Safety Data Sheet Schaudinn’s Fixative
3Proc Natl Acad Sci U.S.A. 1989 May;86(9):3041-4
4Leica Biosystems: Fixation and Fixatives (3) – Fixing Agents Other than the Common Aldehydes (accessed 18 March 2015)
5Davis, B. L. Control of Vapors in a Lab Setting: Sulfur Dioxide; Industrial Hygiene Report for Sentry Air Systems: Houston, TX, October 2013

 Made in the USA
Made in the USA
