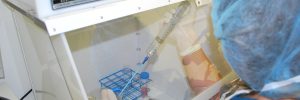
Allergy, ENT, and immunology practices must follow USP 797 when mixing patient-specific allergen extracts for subcutaneous immunotherapy (ACAAI 2023). Section 21 of USP 797 outlines the requirements for compounding allergen extracts to minimize the risk of contamination and ensure the patient’s overall health is protected. USP 797 requirements apply to mixing allergen extracts for… Learn More

 Made in the USA
Made in the USA